Since commercial introduction in the early nineteen nineties, lithium metal batteries (disposable) and lithium-ion batteries (rechargeable) have been produced in their billions. They are the chemistry of choice for cell phones, laptops and electric vehicles due to their high energy storage capabilities, long discharge time and light weight.

However, stories about batteries spontaneously combusting – setting fire to themselves, the device they are in and material nearby have been widely reported in the news.
The likelihood of such an event is extremely low, but there is nothing like a car on fire or a blazing laptop when journalists need a headline. Boeing’s decision in 2014 to ground all its 787s due to issues with lithium batteries was also hard to miss.
In reality, they are often safer than other power options. As The Economist reported, “… with some 30,000 [lithium battery powered] Tesla cars now on the road, fires have affected one in 10,000 vehicles—which sounds bad, but the equivalent statistic for petrol-powered cars is one in 1,300”.
Furthermore, there have been very few deaths related to malfunctioning lithium-ion batteries, while the automobile is responsible for well over 30,000 fatalities a year in the United States alone.
This said, dangers do exist. Anybody responsible for health and safety needs to consider these – especially in environments where zero fires are the aim, such as in air transportation or zones with flammable chemicals. This has lead to restrictions on what lithium batteries you can travel with by air and in how lithium batteries can be shipped.
Thermal runaway in lithium metal and lithium-ion batteries

The main danger lies in a process known as thermal runaway – often referred to as venting with flame and rapid disassembly. This is where an internal short occurs inside the battery causing it to start discharging rapidly. In many cases involving lithium-ion – the battery is charged incorrectly.
Both processes generate heat which can reach temperatures high enough to cause the battery case or surrounding materials to catch fire. In battery packs containing multiple lithium cells the heat from one malfunctioning cell can spread to the next cell causing material failures which lead it to short out and go into thermal runaway.
Thermal runaway in battery packs has been known to occur over very short periods of time leading to explosions and also over many hours as the domino effect spreads from one cell to another where the temperature is never high enough to combust. In this later case, smoke and toxic fumes are still released.
Thermal runaway has a greater chance of happening in lithium based batteries because:
- the high precision with which the battery is made makes shorting more likely than with other chemistries. The separator which keeps the positive cathode from touching the negative anode plate can be less than half the width of a human hair. As such minute pieces of loose metal, invisible to the eye, can end up inside the battery even in the cleanest of manufacturing environments. At any moment they can cause a short.
- lithium-ion batteries require specialty chargers that deliver different power at different times. In the case of battery packs, it may have to balance across many cells.
Design changes to resolve thermal runaway
As the issue cannot be resolved completely during manufacture, concepts have been introduced to ensure cell failure does not lead to thermal runaway. Requirements for individual cells and lithium battery packs in the US are covered by the International Electrotechnical Commission publication IEC 62133. Below, we will cover the general design improvements.
Note not all lithium-ion batteries have all the following features and it is questionable if many cheaper unbranded products have any. Hence, the advice you will receive everywhere is to buy branded batteries recommended by the device’s manufacturer to minimize the risk of thermal runaway.
Venting
Donal Finegan from University College London, who is studying lithium cell failure, explains “when heat cannot escape as fast as it is being generated, this is a runaway reaction that cannot be stopped”. Helping the heat escape limits how hot the cell gets. To achieve this, safety valves are often fitted, which open if internal pressure caused by high temperatures becomes excessive.
CID (circuit interrupt device)
This is any mechanism which shuts down the cell in certain circumstances by disconnecting the terminals from the internal battery components.
It is sometimes a piece of material whose shape changes, causing a disconnection with the terminal. It can also be combined with venting so that if the pressure build up becomes excessive (above 145 psi), it is the vent that not only lets the pressure out, but also disconnects the terminal. This is the case in Diagram 1 below.
In this design, the vent is the negative plate of the cell. When pressure builds up,the negative plate moves outward to let the gas release, while at the same time disconnecting itself from the inner battery. If the heat is being caused by overcharging, this action breaks the circuit and removes the cause before thermal runaway can take hold.
Separator melt down design
The separator between the positive and negative plates in a lithium is porous allowing ions to flow between the two. Some separators have been created which become non-porous by melting down at a specific temperature. This stops the chemical reaction (overcharge or overdischarge) within the battery and should halt heat generation or reduce it where there is a large short caused by metal particles.
On the downside, this renders the battery useless compared to other safety features which essentially ‘reset’ after the issue is resolved (such as incorrect charging) is stopped.
A positive temperature coefficient (PTC)
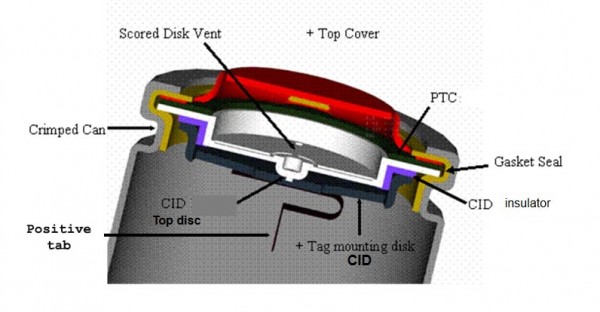
This is a disc of material placed within the battery itself which increases in resistance when current rises over a certain level. The PTC is not sensitive to the current but to temperature levels which would be caused by excessive current.
In doing so, it effectively breaks the circuit and shuts the battery down. As the current drops, its resistance decreases and the battery becomes usable again.
It’s main purpose is to avoid the possibility of thermal runaway caused by overcharging the cell.
Diagram 3 shows a common position of the PTC within a lithium cell placed between the top cover (positive terminal) and the tab which would connect to the positive cathode. For a full diagram of the battery see What are Lithium-Ion batteries?
Protection Circuit Board (PCB)

This is a circuit board designed to protect against over discharge, over charge and shorting out by disconnecting the internal battery from the terminals.
The circuit needs to be attached to both the positive and negative terminals as it requires power to operate ,but this is fractional and a typical PCB would take decades to run a battery flat.
The PCB is made up of a controller which monitors the battery and a switch which disconnects the terminals from the internal cell.
Image 1 shows a PCB and how it is attached to a lithium-ion cell.
In a cell with external foil wrapping it is often difficult to see whether or not it has a PCB, but this article contains an array of tips on how to know.
The PCB brings debate about what a protected battery is. Some argue that a lithium cell or pack is protected if it has a Circuit Interrupt Device (CID), venting or a Positive Temperature Coefficient (PTC) as detailed above. Others suggest it is not ‘fully’ protected unless it has a Protection Circuit Board (PCB).
The multiple safety device approach
Reputable manufacturers will usually use more than one method in order to increase safety. Below is an example of elements that are often added to a good quality lithium battery.
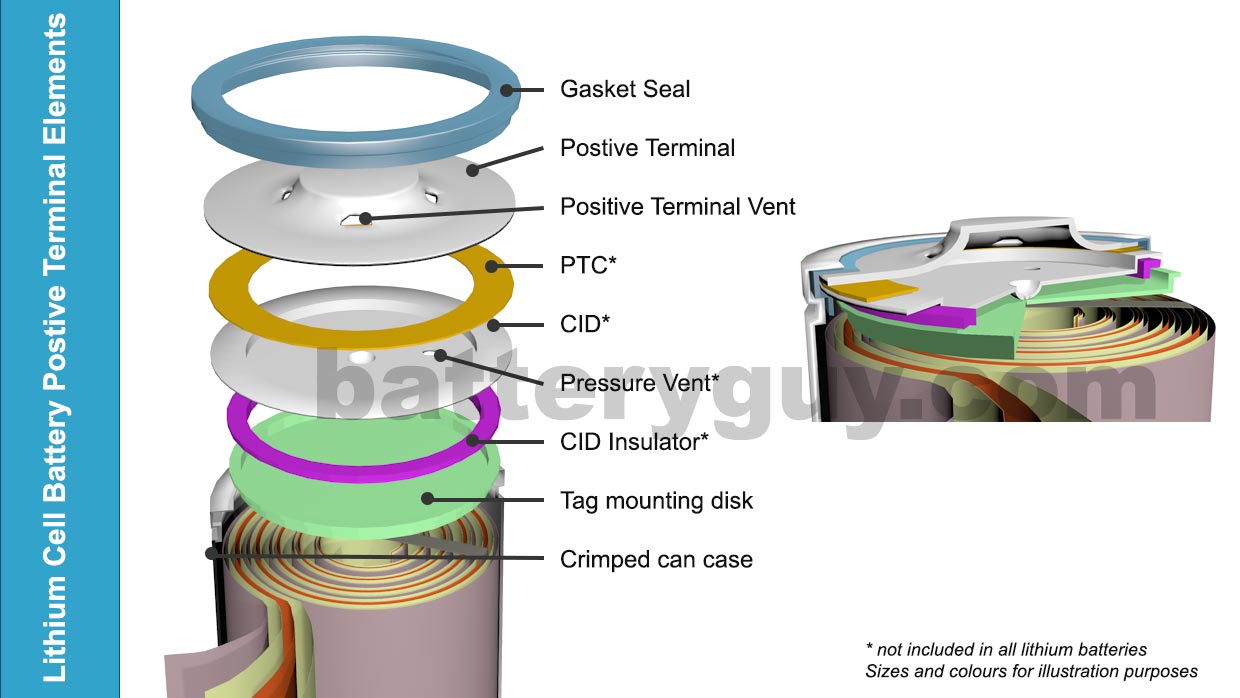
- Gasket Seal – the negative terminal is often connected directly to the battery case in effect making the entire battery case the negative terminal. The gasket seal separates the case from the positive terminal
- Positive Terminal Vents – these are simply holes in the positive terminal to let out any gases that are realeased by the pressure vent in the CID (see below)
- PTC (Positive Temperature Coefficient also sometimes referred to as the “Pressure, Temperature, Current Switch“) – if the battery becomes excessively hot, this material increases in resistance, effectively cutting the positive terminal off from the battery.
- CID ( Circuit Interrupt Device ) – a metal alloy element that changes shape when the temperature rises above a certain point and by doing so cuts the positive terminal off from the battery.
- Pressure Vent – malfunctioning batteries can generate large volumes of hot gases, which if not released, can cause fire or an explosion.
- CID Insulator – makes sure the tag mounting disk does not touch the Circuit Interrupt Device anywhere except at the point designed to change shape at high temperatures
- Tag mounting disk – ensures a strong connection with the positive tab.
Theoretically, a Protection Circuit Board can also be added externally in order to gain yet another layer of protection.
Managing thermal runaway
As Jim McDowall from Saft America puts it, “Do everything possible to eliminate a particular safety event, and then assume it will happen. ” In other words you can manufacture in a clean environment and add some or all of the safety features mentioned above, but then assume the battery and all the safety features will fail.
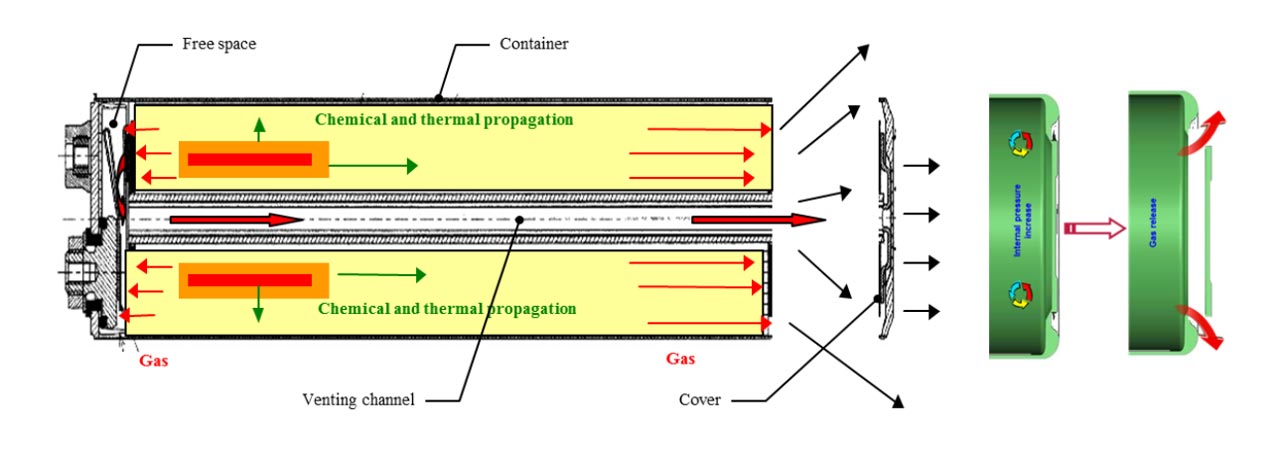
The question then becomes damage limitation, taking two forms:
- Containment – ensuring that should a cell overheat, or even catch fire, it is contained in a way so the fire and heat cannot spread to other surrounding materials.
- Insulation – isolating each cell so that surrounding cells are not affected by a failing cell causing their materials to break down and in turn short out and overheat.
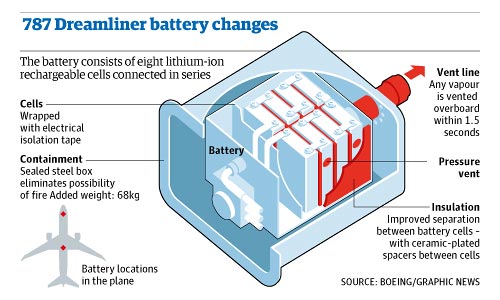
This was part of the fix used by Boeing as can be seen in Diagram 2. The airplane maker used a venting system to remove hot gases fast and insulation to stop heat being transferred from a malfunctioning cell to a healthy one. On top of this, the battery packs were encased in steel boxes so any heat would not cause other nearby materials to catch fire.
The design changes were not ideal because the steel boxes added 68 kilograms of weight to the airplane, but still offered a better power/weight ratio than other battery technologies.
Safety in modern lithium metal and lithium-ion batteries
This array of features and methods make today’s lithium batteries much safer, however many restrictions regarding their use and movement (either shipping or as personal luggage) still remain in place for three reasons:
- lithium is still evolving – manufacturer’s are continuously trying to push the bounds of this chemistry to offer greater power at lighter weights. Unfortunately this does not always work out as the Samsung Galaxy Note issues of 2016 demonstrated.
- there are still a large number of unbranded batteries manufactured today with little or substandard safety features. They are widely available and popular because of their low price compared to purchasing original equipment branded replacements. Many make their way into the likes of laptops and cell phones. For transport companies or airline operators, it is difficult to differentiate between these leading to blanket bans or restrictions.
- the legacy of earlier technology lives on in people’s minds such as the recall of 100,000 notebooks by Sony, HP, Dell and Toshiba in 2008 after more than a dozen cases where laptops burst into flames and two people suffered minor burns.


Leave A Comment?