
Lithium ion has gone from virtually unheard of in 1990 to one of the most used battery chemistries in the world today. Its popularity is driven by the high power to weight ratio that it offers and its ability to discharge and recharge many more times than other battery alternatives.
In industry lingo that translates to a high specific energy and an excellent cycle life.
With Lithium, keep in mind, the moment lithium ion batteries run flat and are plugged in to charge, they will never reach their original capacity. Gradually over successive cycles the capacity will reduce until the battery can no longer power the application it was designed for. So what happens? To find out, first we need to understand what happens inside the battery.
How a lithium ion battery works
The basic principles of a lithium ion battery are as follows –
- When the battery is fully charged the negative electrode is full of lithium ions.
- As soon as it is connected to an appliance these lithium ions begin to move through the electrolyte to the positive electrode and this chemical reaction creates the electrical charge at the terminals.
- When there are no more lithium ions left on the negative plate the battery is flat and recharging is needed to reverse the process.
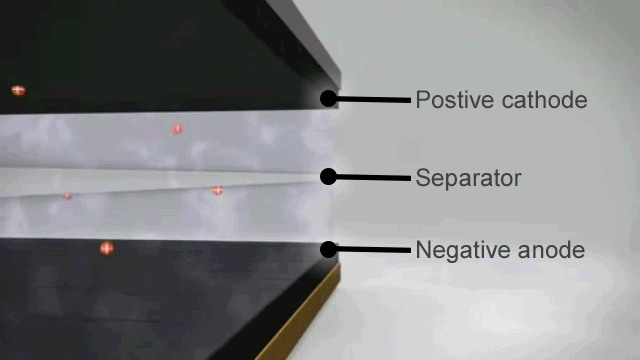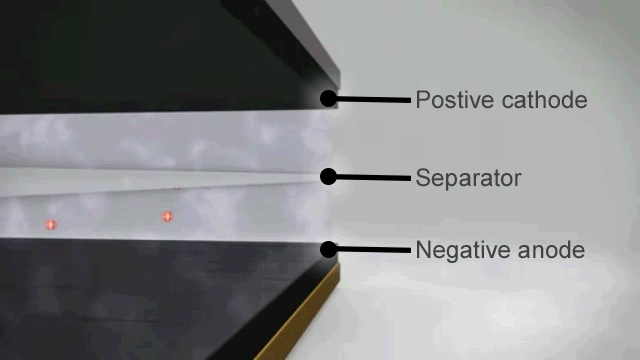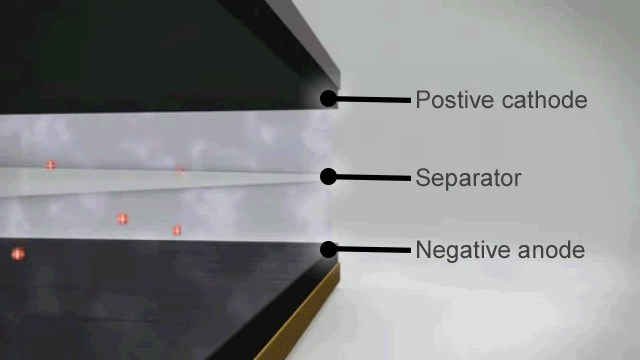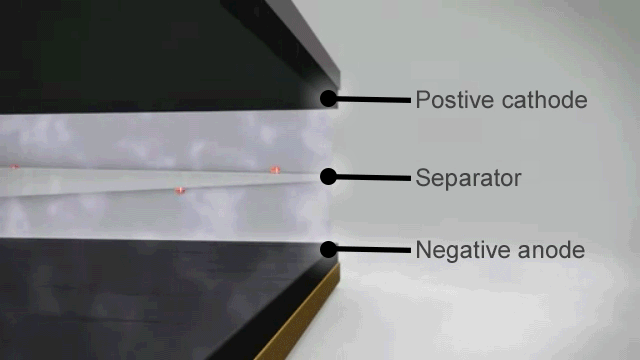
In theory that should mean that lithium ion batteries can discharge and recharge forever, but in reality the process is not quite perfect. From the very first discharge and recharge cycle the capacity of the battery degrades.
There are four issues at play:
- Solid Electrolyte Interface build up on the negative electrode
- Electrolyte oxidation on the positive electrode
- Lithium plating
- Mechanical degradation
The Solid Electrolyte Interface and electrolyte oxidation
While a lithium ion battery is being charged lithium oxide and lithium carbonate atoms form a film on the negative electrode known as a Solid Electrolyte Interface (SEI).
A similar film known as electrolyte oxidation occurs on the positive electrode if the battery is operated at a full or near full state of charge in temperatures of 104°F (40°C ) or above. Electrolyte oxidation is nothing new, the process is actively used to treat metals so that they do not rust. This is very popular in the yachting industry, but not welcome in a battery.
This heat may sound rare, but operating temperatures inside devices such as laptops can frequently go far above this limit. Its also common in some parts of the world as Nissan found out when their Leaf electric car owners living in hot areas such as Arizona sued because of poor battery capacity after relatively few discharge and recharge cycles.
Electrolyte oxidation also increases self-discharge rates which can explain why some batteries seem to need regular charging even when not in use.
These films are initially so thin that most lithium ions can pass through, but after progressive charging and discharging cycles, they gradually thicken. Each time the film thickens it reduces the number of lithium ions that can move between the plates, affecting the ability of the battery to fully charge as well as increasing the time it takes to recharge.
Various manufacturers have been able to reduce the build up of one or either films with the addition of certain chemicals to the electrolyte, but no one has managed to stop the process altogether.
Lithium plating (aka – Lithium deposition)
When lithium ion batteries are charged rapidly the rate at which ions are leaving the positive electrode and heading towards the negative electrode exceeds the rate at which the negative electrode can absorb the ions. In such cases the ions then become a metallic deposit on the electrode.
The positive electrode is especially poor at absorbing ions at low temperatures, so mix rapid charging and a cold environment and you get a recipe for rapid lithium plating.
Less ions around means less battery capacity. In more extreme circumstances the build up can cause the battery to short out and fail completely.
The video below shows plating taking place. Note that it starts gradually, but you can see it rapidly taking place from about the 30 second mark.
Mechanical degradation
There’s a lot of physical changes going on inside a lithium-ion battery that can naturally cause strains on materials that are often manufactured to widths thinner than a human hair. Researches have noticed a variety of results from these stresses such as cracking of the electrodes.
Some mechanical degradation only reduces the batteries ability to charge and discharge whereas other forms or extremes can cause it to fail completely.
Not all lithium ion batteries are the same
There are a wealth of lithium ion battery types (see What are lithium ion batteries for a list of the more popular variations) in use today using slightly different material and chemical make ups.
Lithium-Manganese Oxide, for example, is highly susceptible to Solid Electrolyte Interface, while Lithium Titanate is a far better alternative (but costs significantly more to produce).
Ultimately, all lithium-ion batteries suffer all four of the issues covered in this article and for these reasons all lithium ion batteries slowly degrade and eventually die.
How to handle ‘dead’ lithium ion batteries
When your lithium ion battery appears to be at the end of its life there is a temptation to view it as completely dead, but in reality units often still have power in them – just not enough to power the device they were intended for. The remaining energy can still be enough to start fires and explosions if the terminals are shorted out, say by placing the exposed battery in a drawer full of keys.
Its for this reason that shipping companies treat the transportation of old lithium ion exactly the same way as they treat new units and why everyone should treat exhausted or new batteries as the same when it comes to storage and handling.


Hi and thanks for your website,
Do b Li batteries such as the ones in ebikes give out full power until they are fully discharged or does the power output reduce as it reaches but before it is empty?
Generally lithium batteries are very good at keeping a constant power output until they are close to full discharge.