Lithium-ion refers to rechargeable (or secondary) lithium batteries. They should not be confused with lithium metal disposable batteries which we deal with in the article What are Lithium metal batteries.
The field of Lithium-Ion batteries is a fast moving one with new variations based on slightly different chemistries becoming available ever more frequently. At the same time, the actual methods of construction are also evolving to allow greater flexibility in shapes as well as literal flexibility so units can bend without damage.
Basic structure of a Lithium-Ion battery
At a very basic level a Lithium Ion battery is made up of:
- An anode positive plate.
- A cathode negative plate
- A separator to ensure the plates do not touch but porous enough to allow chemical reactions between the two via an electrolyte solution
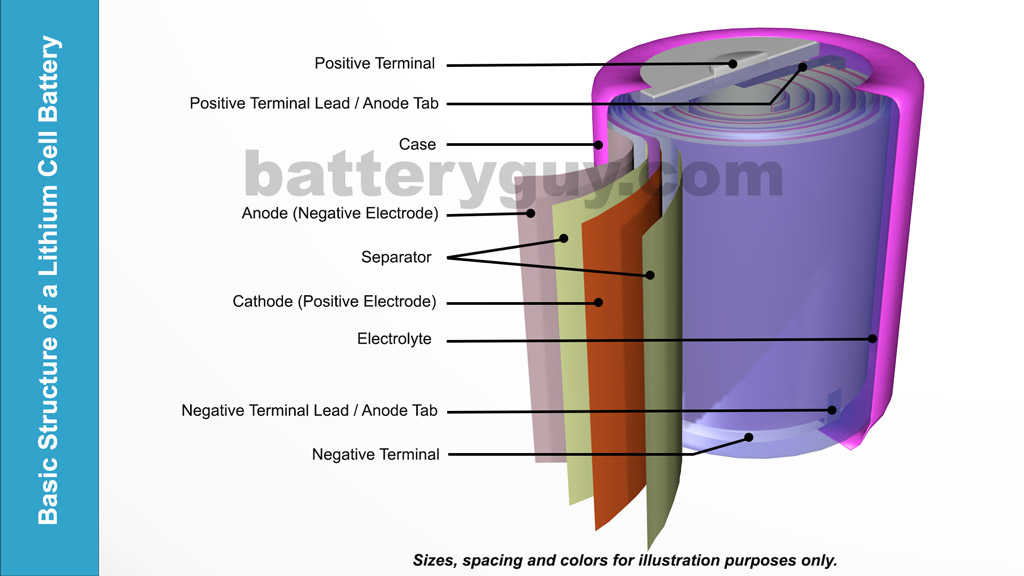
The above picture is for illustration only and it should be noted that the plates and separator are usually wound much tighter while the electrolyte is sometimes simply impregnated into the separator or can itself be a dry powder.
(Video of How a Lithium Battery is Made with Transcript)
The anode, cathode and separator are manufactured with extreme precision. The more anode and cathode in the battery, the more energy it can deliver and so the separator can be no more than 25 µm thick. That’s about half the thickness of a human hair.
Manufacturing process of a Lithium-Ion Battery
1) In some manufacturing processes the separator is soaked with electrolyte before being wound
If you would like to understand the manufacturing process better there is an excellent blog post written by Nathan Seidle where he documents his tour down a Lithium Polymer production line in a Chinese factory.
Lithium-Ion battery sizes

While the manufacturing process flowchart above can convey the many forms and shapes a lithium-ion battery can take on, it does not reflect the immense diversity of sizes that the battery can come in. At the extra tiny end of the scale are batteries less than 0.5mm thick which can be used as an internal part of a credit card. On the larger side of things cells the size of a bricks are manufactured and linked together in battery banks to power buses.
In short, lithium-ion offers a rechargeable battery in almost infinite shapes and sizes with terminals wherever needed and in a variety of forms.
Additional elements in lithium-ion batteries
The basic structure of a lithium-ion battery above shows the parts needed to make the battery function in commercial applications, but a number of other elements are often added. These are designed to avoid fire or explosion caused by manufacturing defects or abuse such as incorrect charging (see Safety issues with lithium batteries).
The image below shows these extra additions:
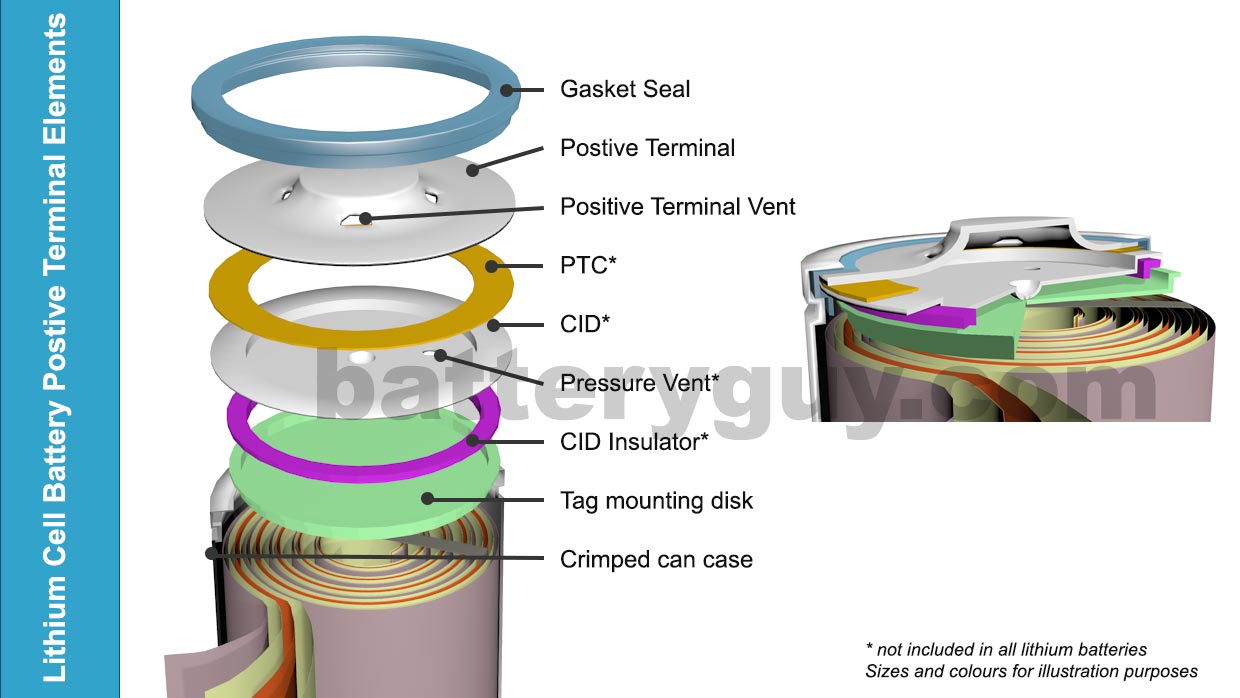
- Gasket Seal – the negative terminal is often connected directly to the battery case in effect making the entire battery case the negative terminal. The gasket seal separates the case from the positive terminal
- Positive Terminal Vents – these are simply holes in the positive terminal to let out any gases that are released by the pressure vent in the CID (see below)
- PTC (Positive Temperature Coefficient also sometimes referred to as the “Pressure, Temperature, Current Switch“) – if the battery becomes excessively hot this material increases in resistance effectively cutting the positive terminal off from the battery.
- CID ( Circuit Interrupt Device ) – a metal alloy element that changes shape when the temperature rises above a certain point and by doing so disconnects the positive terminal from the battery.
- Pressure Vent – malfunctioning batteries can generate large volumes of hot gases which, if not released, can cause fire or an explosion.
- CID Insulator – ensures the tag mounting disk does not touch the Circuit Interrupt Device anywhere except at the point designed to change shape at high temperatures
- Tag mounting disk – ensures a strong connection with the positive tab.
It should be noted that a large number of unbranded lithium batteries sold at low prices do not contain these elements. Recognized brand names will be able to provide details regarding which elements they include on a battery by battery basis.
Evolution of Lithium-Ion batteries
Since 1991 six types of Lithium-Ion battery types have entered the mainstream market:
- 1991 – Lithium Cobalt Oxide (Li-cobalt or LCO ) – with an energy to weight ratio of 150-200Wh/kg this is the battery of choice for smart phones, laptops and cameras even though they cannot be fast charged and have a short lifespan.
- 1996 – Lithium Manganese Oxide (Li-manganese or LMO) – a safer battery but with a shorter calendar and cycle life. However it is better at delivering high current, making it popular in electric vehicles and power tools.
- 1996 – Lithium Iron Phosphate (Li-phosphate or LFP) – a very safe battery, even when abused, with a long cycle life and the ability to produce high currents, but the shortest calendar life of lithium-ion batteries. Often used as a replacement to starter batteries in hybrid cars or cars fitted with engines that shut down when stationary.
- 1999 – Lithium Nickel Cobalt Aluminum Oxide (Li-aluminum or NCA) – offers an outstanding energy to weight ratio, but costly to produce and with a very short cycle life it is mainly limited to specific industrial and medical applications.
- 1999 – Lithium Titanate ( Li-titanate or LTO) – has excellent discharge capabilities and operates well at low temperatures (up to 80% capacity at -22°F (-30°C), but high manufacturing costs and a low energy to weight ratio limit its use to certain types of Uninterruptible Power Supplies (UPS) and solar powered applications such as street lighting.
- 2008 – Lithium Nickel Manganese Cobalt Oxide (NMC) – a long lasting battery that can produce the high power needed for power tools and smaller electric vehicles, but with a mid-range energy to weight ratio.
This timeline does not reflect constant improvements, but does show where the introduction of different lithium batteries over time has often been based on a needed specific outcome such as lighter weight or the ability to operate at particular temperatures.
While chemistries can vary, so can the actual physical construction of the battery, as can be seen in the manufacturing process above. The three most well known types are:
- Cylinder – the plates are wound into a circular shape (known as the jelly roll or Swiss roll) with terminals on the top and bottom.
- Can – the plates are wound into a semi-rectangular shape so the battery can be wide, but flatter than a cylinder for use in applications such as cell phones and tablets.
- Pouch – the plates are placed in a flexible foil container that can often literally bend to fit complex spaces.
Thus, one of the most important advantages Lithium has over many other battery types is its ability to be manufactured into specific shapes or even structures that do not have a solid shape and can flex as required without damaging the internal components.
Comparison of Lithium-Ion battery types
There are two key types of Lithium-ion batteries:
- Cobalt based – excellent at delivering low energy over long periods. The chemistry of choice in cell phones, laptops, cameras, etc.
- Manganese based – better at delivering high current fast and so used for applications such as power tools and electric vehicles. When used in applications such as laptops however, they last half as long as their cobalt counterparts.
Lithium-ion development has centered around trying to find one cell that could cover both long discharges as well as high energy on demand by adding metals such as iron, aluminum and nickel.
| Type | Voltage (operating range) |
Specific Energy | Specific Capacity | Cycle Life | Calendar Life | Fast Charge |
|---|---|---|---|---|---|---|
| Lithium Cobalt Oxide | 3.6 (3 – 4.2 volts) |
150-200 Wh/kg | 140 mAh/g | 500 – 1,0001 | ?2 | No |
| Lithium Manganese Oxide | 3.7 (3 – 4.2 volts) |
100-150 Wh/kg | 125 mAh/g | 300-7001 | ?2 | Yes |
| Lithium Iron Phosphate | 3.2 (2.5 – 3.6 volts) |
90-120 Wh/kg | 134 mAh/g | 1,000 – 2,0001 | ?2 | ?2 |
| Lithium Nickel Cobalt Aluminum Oxide | 3.6 (3 – 4.2 volts) |
200-260 Wh/kg | 180 mAh/g | 5001 | ?2 | ?2 |
| Lithium Titanate | 2.4 (1.8 – 2.85 volts) |
70-80 Wh/kg | 150 mAh/g | 3,000 – 7,000 | 15 years | Yes |
| Lithium Nickel Manganese Cobalt Oxide | 3.6 (3 – 4.2 volts) |
120-220 Wh/kg | 200 mAh/g | 1,000 – 2,0001 | 20 years | ? |
|
||||||
While there appear to be clearly superior options in terms of capacity (energy/weight ratios) and life cycles, engineers also need to take into account other factors such as safety, number of cycles, or the batteries ability to fast charge. As such, which battery to choose depends greatly on the requirements of the device. A battery for a pacemaker for example, needs to deliver consistent energy, last a long time and be safe – these three requirements ranking far higher than other needs.
However, the figures above should also be treated with caution, as different manufacturers using what they claim to be the same mix of chemistries produce batteries with different abilities. For example in March 2015, Andreas Gutsch of the Karlsruhe Institute of Technology published results of a test into Lithium-Ion life cycles and found that after 1,000 cycles, some cells lost up to 30% of their capacity – i.e. it was not possible to fully recharge them. Results varied by manufacturer and country of origin even though the battery types were theoretically identical.
In applications such as electric cars, this can make a big difference in terms of how far you can travel on one charge and how far you can travel over a batteries life cycle. A battery at full capacity may be able to power an electric car for 500 kilometers. At 70% capacity, that figure drops to 350 kilometers. Over several hundred charges and discharges, this adds up to a difference of thousands of kilometers.
Similar debates surround calendar life, because many lithium-ion technologies are too new to prove such figures consistently.
With this in mind, the following table ranks from ‘best’ at the top to ‘worst’ at the bottom based on lithium-ion battery types for a number of characteristics using data currently available and assuming the battery is sourced from a reputable manufacturer. Where more than one battery type is shown, this means that their performance is broadly similar.
| Specific Energy | Specific Capacity | Cost | Safety | Cycle Life |
|---|---|---|---|---|
| Lithium Nickel Cobalt Aluminum Oxide | Lithium Nickel Manganese Cobalt Oxide | Lithium Cobalt Oxide | Lithium Titanate | Lithium Titanate |
| Lithium Cobalt Oxide | Lithium Nickel Cobalt Aluminum Oxide | Lithium Manganese Oxide | Lithium Iron Phosphate | Lithium Nickel Manganese Cobalt Oxide |
| Lithium Nickel Manganese Cobalt Oxide | Lithium Titanate | Lithium Nickel Manganese Cobalt Oxide | Lithium Manganese Oxide | Lithium Iron Phosphate |
| Lithium Manganese Oxide | Lithium Cobalt Oxide | Lithium Iron Phosphate | Lithium Nickel Manganese Cobalt Oxide | Lithium Cobalt Oxide |
| Lithium Iron Phosphate | Lithium Iron Phosphate | Lithium Nickel Cobalt Aluminum Oxide | Lithium Nickel Cobalt Aluminum Oxide | Lithium Manganese Oxide |
| Lithium Titanate | Lithium Manganese Oxide | Lithium Titanate | Lithium Cobalt Oxide | Lithium Nickel Cobalt Aluminum Oxide |
Lithium-Ion versus other battery chemistries
When lithium metal (disposable) batteries first became commercially available in the 1970s, most portable devices were powered by nickel cadmium batteries. Rechargeable lithium-ion batteries did not make their debut until the early 1990s, but since then they have not stopped evolving.
They have a number of advantageous over other battery chemistries:
- they can store over four times as much energy as lead acid batteries and twice as much energy as nickel based.
- not only can they store more, they can do it in less space giving them double the energy density of nickel cadmium.
- they are low maintenance and easier to store with a much smaller self discharge rate than nickel cadmium.
- a lithium-ion cell produces 3.6 volts, three times higher than the nickel cadmium cells 1.2 volts.
- from an environmental point of view, they are less damaging if disposed of incorrectly
But it is not all a one way street. Lithium-ion has been plagued by safety issues. Most notably thermal runaway – when a cell shorts out or is incorrectly charged, extreme heats are generated which can start fires and YouTube is not short of examples to demonstrate this issue! As such, modern Lithium-ion cells must be fitted with features which add to weight and cost. Given that, lithium was already a more expensive battery compared to nickel cadmium. These requirements now make it nearly 50% more costly to manufacture.
The thermal runaway issues have also lead to the introduction of air travel restrictions and shipping regulations which makes commercial shipping more of a headache
The overall life of lithium-ion has also not been particularly encouraging with most not lasting more than 36 months. In many applications such as smartphones, where consumers frequently upgrade, this is not noticeable. With commercial or industrial applications and increasing usage in vehicles, this is a drawback.
The technology remains fast moving with new types coming onto the market every year. Many have no track record regarding stability, calendar life and questionable cycle life abilities without losing capacity. By the time these are assessed in real world environments, certain models are often superseded and many safety issues only come to the fore when in use as the 2016 Samsung Galaxy Note issues demonstrated.






Leave A Comment?