When we look at early batteries and compare them with what powers our portable products of today like cell phones or laptops it’s tempting to think battery technology has progressed from basic to hi-tech in easy-to-follow steps.
But are the batteries which have been invented over the last few years really better in every way to the bulky efforts of our ancestors? Everything is not as it might seem.
The first battery with any kind of practical application was the trough battery which appeared in 1801. If you open up the starter battery of a gas or diesel car and compared it with a trough battery from over two hundred years ago you would immediately notice the similarities.
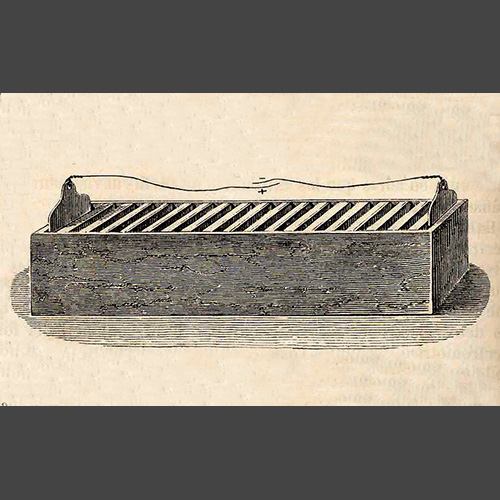
Despite decades of technological breakthroughs our automotive engines still use a design that dates back more than two centuries. How come?
To understand this we need to consider each ‘breakthrough’ along with its pros and cons.
The first 100 years of battery design
The 1800s were mostly about improving the trough battery so it could be used in different applications without acid leaking all over the place. In 1859 a rechargeable version appeared for the first time and over the last part of the century other features were added that made it more powerful and effective.
Ultimately however it was still based on the same principle – lead plates immersed in acid from which they took on the name Lead Acid Batteries.
The first non lead acid battery
It wasn’t until 1886 that something different appeared – a dry cell battery manufactured using zinc and carbon. It was lighter, smaller and did not require any maintenance such as the need to top up the electrolyte liquid used in the lead acid design.
Zinc carbon allowed the invention of handheld battery powered products such as the flashlight. A lead acid battery would be completely impractical for this purpose because acid would leak whenever the flashlight as held at a tilt. Furthermore, lead and acid are heavy, not exactly portable.

But zinc carbon did not make the lead acid battery into an instant museum piece. Despite its obvious advantages it was not rechargeable and had a much smaller power output. It was also more fiddly to manufacture, and so more costly, compared to lead acid.
Zinc-carbon had a role but it did not replace lead acid, especially in an age where the first automotive cars were beginning to appear.
Nickel iron appears as a possible lead acid replacement
In 1903 Thomas Edison, inventor of the light bulb some 20 years earlier, patented a battery based on nickel and iron. It was superior to lead acid in that it could withstand knocks and vibration far better than lead acid.
Lead acid battery plates are covered in a paste which is easily damaged if the battery is not kept on a stable platform or in a static environment.
Nickel iron batteries didn’t have these issues and Edison hoped they would become the power choice for all cars, many of which were all electric at the time.
Despite these advantages nickel iron was more expensive to manufacture and had to be recharged regularly, even when the battery wasn’t being used.

They also did not perform well in the very hot or cold environments which could be found in much of North America throughout the year.
Nickel iron would eventually find a use in the mining industry where their bulky nature was not an issue, temperatures were consistent, and their vibration resistance was an asset in drilling machinery. This would remain their niche to this day.
Nickel Cadmium challenges Zinc Carbon
In 1946 batteries based on Nickel and Cadmium appeared. They had been invented almost 50 years earlier but taking the design from concept to production had proved difficult.
Nickel cadmium (or NiCad for short) batteries were similar to zinc carbon. Lightweight, small and maintenance free. Their main advantage over zinc carbon was that they were rechargeable. These advantages however, came at a cost which meant although they took a large share of the zinc carbon market, they did not replace it altogether.

As with zinc carbon, they could not offer the power surges needed to start an automotive engine and their production costs meant that for anyone who did not need smaller and lighter, lead acid remained the more economical choice.
NiCad as a design would have a limited life. Cadmium is highly toxic and after it was discovered that used batteries leaked, poisoning ground water sources, they were banned by many countries from the 1980s onward.
What had looked like a technological advance turned out to be an environmental hazard.
Alkaline improves on zinc carbon
In 1955 Lew Urry invented a battery based on alkaline. Like zinc-carbon it was small, light and disposable. It’s advantage over zinc-carbon was that it lasted ten times longer but with a higher price tag.
Despite the cost it was still more economical than zinc-carbon because of its longer life. Zinc carbon sales would drop dramatically but it was not obsolete. For those who wanted “small, light, now and cheap” zinc carbon remained the answer. This type of demand was often created by the manufacturers of portable electrical products who wanted to market their goods as “batteries included”.

With alkaline’s high production costs and their inability to recharge, alkaline batteries posed no threat to the lead acid market which continued to grow rapidly as the automotive industry expanded exponentially.
Lead acid evolves.
The 1970s saw a lead acid breakthrough. Lead acid’s major flaw was the acid. In liquid form this could easily spill if the battery was tipped at an angle or the case was broken by an impact.
In 1972 Enersys Cyclon started to produce a lead acid battery where the acid was held between the lead plates in a material known as an Absorbent Glass Mat, or AGM for short.
This allowed for smaller lead acid batteries that could be used at any angle without the danger of acid spillage. The design also meant acid did not evaporate and need to be regularly topped up. As such the batteries could be permanently sealed during production.
The closed nature of the battery gave rise to the term ‘Sealed Lead Acid’ or SLA for short.
Now there was a lead acid battery that could compete with NiCad, the only other truly lightweight battery that was rechargeable.

But it’s higher costs and greater weight, with no other major advantages, meant it was no serious competitor to NiCad.
This first SLA battery would eventually find a niche in applications such as emergency lighting but it would have no major impact on the landscape of portable power.
Moreover, those industries for which its advantages had no bearing, such as automotive starter batteries, would continue to use the lower cost lead acid design which now became known as ‘flooded lead acid’ to reflect the liquid nature of the acidic electrolyte.
Lightweight batteries for handheld devices improve
In 1973 Duracell launched a mercury based battery to the public. Duracell had been growing for several decades as a manufacturer of alkaline batteries, those longer lasting cells compared to zinc carbon.
Duracell had been developing mercury batteries for many years and the had proved popular in military applications such as radios because they could operate at much higher and lower temperatures compared to zinc carbon or alkaline.
They could also provide bursts of high energy, something only lead acid had been capable of, but production costs were so high and the fact that the design was non-rechargeable meant there was no real chance that they would replace lead acid.
Higher manufacturing costs for features the general public did not need kept mercury as a portable power product with a niche market.

But by the 1970s two things had changed. Duracell had managed to reduce manufacturing costs significantly and flash photography was emerging as a market which needed exactly the type of high energy bursts that mercury batteries could provide.
Duracell’s mercury battery would find a niche alongside zinc carbon and alkaline but it was still a disposable battery and so no answer to the very light and rechargeable NiCad or the low cost flooded lead acid.
Portable … and truly portable
By now the battery market was divided into two main areas. Those that were small, light, portable and able to provide a constant power output over long periods of time – perfect for radios, flashlights and the like.
And on the other side those that were cheaper, larger, heavier and bulkier but could provide strong current in short periods as needed by automotive vehicles to start gas and diesel engines. Here no technological advances were better than lead acid despite its base design already being more than 150 years old.
But new markets were emerging – leisure and emergency lighting. Both caravaners and commercial venues needed a low cost battery that would provide consistent power over long periods of time to either power the mobile holiday home or, in the case of commercial venues, emergency lighting.
They also needed these batteries to be rechargeable leaving only lead acid or nickel cadmium as possibilities. Nickel cadmium and AGM lead acid costs were high but long, slow discharges damaged flooded lead acid batteries.
This process caused their internal plates to buckle which either reduced their lifespan or shorted them out completely. As weight was not a major issue the answer came initially through thicker plates and these batteries became known as ‘deep cycle lead acid’ or ‘leisure batteries’.
They could provide power slowly and consistently to a deeper level of discharge compared to standard flooded lead acid – which was now also referred to as ‘cranking’ because it was ideal for turning over engines to get them started. The extra lead in ‘deep cycle’ increased the price but not anywhere near the costs of AGM or NiCad.
There was only one problem. Both types of lead acid batteries, ‘cranking’ and ‘deep cycle’ looked exactly the same and many underhand companies labelled their ‘cranking’ products as deep cycle or leisure to cash in on the higher pricing. This issue still exists today.
Lead acid for sports
The other emerging market of the 1970s was power sports such as off road driving, motor biking or jet skiing. The vibrations and jolts these vehicles experienced were exactly the actions which caused damage to lead acid batteries but no other battery design was able to provide the power needed to start engines.
Thomas Edisons nickel-iron batteries from 1903 were vibration resistant, but they were bulky, discharged when not in use and had poor performance in hot or cold climates.
The AGM concept (which by now could be found in many lead acid products) did not provide an answer either because the glass mats rubbed against the lead plates causing internal damage.
The breakthrough came in 1981 with ‘gel lead acid’. This replaced the liquid acid with a silicone gel which glued itself to the plates holding everything in place no matter the jolts or vibrations experienced.

It was the perfect answer for power sports but it was a costly one with no particular benefits for the traditional automotive and leisure markets so they continued to use the flooded lead acid design.
AGM lead acid costs did however, continue to decrease making them more viable for applications such as emergency lighting and, later, uninterruptible power supplies (UPS).
Lead acid becomes maintenance free
Although the design of flooded lead acid batteries remained fundamentally unchanged this battery type did see incremental improvements. Better manufacturing techniques allowed for ‘maintenance free’ units.
These used one-way vents to let out any dangerous build up of gases but otherwise they were completely sealed. This stopped the acidic electrolyte evaporating and meant regular top ups were no longer required.
These were, like AGM, often referred to as ‘sealed lead acid’ which to this day can cause some confusion.
NiCad collides with the environment
Meanwhile, in the small and light battery market, there was a growing understanding about how damaging NiCad batteries were to the environment. A growing consumption followed by disposal in landfills was revealing how rusting cells were polluting ground water with toxic metals.
The problem was that no rechargeable alternative existed at any reasonable price point. This changed in 1989 when Nickel Metal-Hydride (NiMH) batteries became viable. They had existed in the lab since the late 1960s but did not last long when discharged and recharged.
A new stable design produced a cell that actually lasted longer and performed better than the toxic NiCad. Shortly after its introduction many countries placed outright bans on NiCad bringing its dominance as a small, rechargeable battery to an abrupt end.
Although Nickel Metal-Hydride replaced NiCad in most areas it was still not a cell with the performance needed to start an engine. It’s higher cost, relative to many types of other lead acid batteries, meant it did little to dent demand for lead acid.
Lithium changes the market for handheld devices
In 1991 Sony produced the first Lithium-Ion battery which would be a game changer for handheld devices and the emerging industry of portable computers. It lasted longer, recharged faster and had a greater overall life span than Nickel Metal-Hydride.
Despite these advantages it was extremely expensive to produce due to the micro-technology involved. Over the next two decades advances would be made to reduce these costs drastically but not to the point that it would completely replace Nickel Metal-Hydride.

What Lithium-Ion batteries did exceptionally well was to provide a constant power over long periods. Ideal for mobile phones, laptops and even electric lawnmowers.
What they could not do was provide large amounts of power in small periods of time. This area, essential to the automotive market, remained the domain of lead-acid in its many forms.
There was still nothing as cheap as a flooded lead acid battery due to its simple design and ease of manufacturing and for this reason it is still found, over two hundred years after its first use, underneath the hoods of gas and diesel powered vehicles.
Will electric cars end the lead acid story?
As electric cars, powered by lithium-ion, continue to grow their share of the automotive market might we finally be looking at the end of lead acid? Possibly but question marks remain over electric vehicles that have not yet been fully answered.
The first is how much electricity it takes to build, and then recharge, the batteries. If this comes from coal fired power stations is there any difference to the overall levels of pollution created by electric transport?
Safety concerns also remain as lithium-ion batteries that short out create large amounts of heat which have caused fires and even fatal airplane crashes.

The possible ‘in between’ solution are hybrid vehicles with ‘stop-start’ engines. However these are often based on using two lead acid batteries. One to operate the ‘stop-start’ feature and the other to power on board appliances while the engine is switched off.
If hybrid becomes the dominant vehicle design we might actually see demand for lead acid accelerating rather than declining.
Lithium-ion in a recycling world
The other Achilles heel for Lithium-Ion is exactly the asset which made it a breakthrough – it’s complexity at a micro-level.
Modern lithium batteries can sustain thousands of discharges and recharges (known as ‘cycles’) but like all battery designs before them they do eventually die.
At the same time, it has become accepted thinking that we cannot continue to dig materials out of the ground to make more of everything. The planet only has finite resources and so we will need to recycle more of what we have already manufactured but has reached the end of its life.
Lithium batteries often contain different materials whose width is less than that of a human hair. Extracting these from used lithium cells is time and energy consuming.

Lead acid, on the other hand, is simple to pull a part and separate the lead from the plastic casing.
So where does this leave us in our search for ‘the best battery’. The answer depends on what you need it for.
No ‘perfect’ battery, just different battery designs for different markets
If you need something light, cheap and disposable zinc-carbon foots the bill. For manufacturers of goods who want to say “batteries included” but keep costs low, zinc carbon provides the answer.
If you are prepared to pay a little more and save in the long term alkaline is better than zinc carbon.
For rechargeable and truly portable batteries Nickel Metal-Hydride is still a cost effective alternative to Lithium-Ion because it is easier to manufacture. For this reason you will still see it as the basis for many everyday batteries found at the supermarket checkout.
Where weight and regular recharging is important, such as in laptops and smart phones, lithium-ion leads the field.
But when weight becomes less of a factor the much lower costs of lead acid make it as popular as ever.
As for the question of whether Lithium-Ion might one day take over from lead acid, especially if manufacturing costs continue to reduce?
It’s possible but a need to protect the environment may ironically be its downfall and in fifty years time we might still find the trusty flooded lead acid under the hoods of our cars.
In Summary
What the history of the battery has taught us is not that batteries have continuously evolved and become ‘better’ in the way we see products like computers progressing. It has not been a case that every new design replaced the old one.
More often than not each step forward found, or created, a new market but each step forward also came at a higher production cost. This meant that older designs always retained a market with cost sensitive buyers and it is why the technically inferior zinc carbon disposable battery is still in production today – its cheap.
As for lead acid, it has evolved over the decades with different variants serving different markets but take any lead acid battery apart and what you will find is a design that does not look that much different to the trough battery of 1801.
For certain markets where low costs are important and weight is not a critical factor lead acid is still the ‘perfect’ battery.

