There are three common types of lead acid battery:
- Flooded
- Gel
- Absorbent Glass Mat (AGM)
Note that both Gel and AGM are often simply referred to as Sealed Lead Acid batteries.
The Gel and AGM batteries are a variation on the flooded type so we’ll start there.
Structure of a flooded lead acid battery
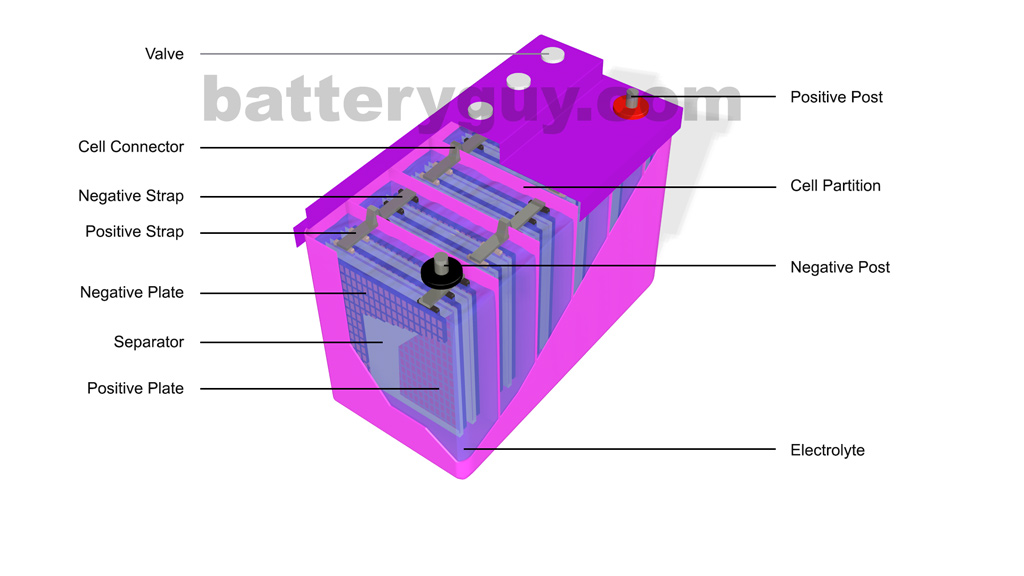
A lead acid battery is made up of eight components
- Positive and negative lead or lead alloy plates
- A lead oxide paste which is applied to the positive plates
- A lead oxide paste with the addition of powdered sulfates which is applied to the negative plates
- Porous separators which stop the negative and positive plates touching each other but allow current to move between them
- Positive straps which connect the positive plates together in each cell and negative straps which connect the negative plates together in each cell
- Cell connectors which join the positive strap of one cell to the negative strap of the next cell
- Electrolyte – either as a solution of water and sulfuric acid or a gel
- A case and lid – normally made from a polypropylene plastic
- Terminal posts (usually lead) to connect the battery to an appliance
Manufacturing process for lead acid batteries
(Video of How a Flooded Lead Acid Battery is made with Transcript)
The process starts with the fabrication of lead plates. In some types of lead acid batteries lead alone is not strong enough and so other metals such as tin are added to give the plate strength. Because the greater the surface area of the plate, the better the capacity of a battery, several types of plate have been developed
The three most common types of plates are:
- grid plates – the plates have a grid like honeycomb type structure, most popular in starter batteries
- grooved plates – the surface of the plates is made up of V shaped grooves
- tubular plates – each plate is made up of one row of tubes side by side providing greater strength and so more popular in deep cycle batteries
The plates do not need to be flat, in some battery types such as those that use Spiral Cell technology they are wound to create a cylindrical shape.
An active material is needed on the lead plates. This is lead oxide (powdered lead and other materials) on the positive plates and lead oxide with powdered sulfates on the negative plates. The active material is usually made into a paste by adding sulfuric acid and water. The paste acts like a sponge soaking up the electrolyte that is added later and keeping this electrolyte close to the plates to improve the battery’s performance.

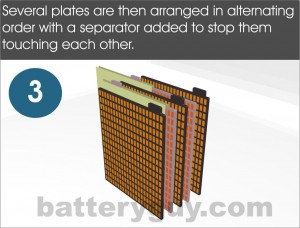
The plates are then assembled in alternating order – positive, negative, positive, negative, etc. Positive plates are always positioned between two negative plates so in any assembly there will always be one more negative plate than positive. A porous separator is placed between the plates to avoid them touching which would cause them to short out and kill off the battery. Two metal elements are then added – one connects all the positive plates together (the positive strap) and one connects all the negative plates (the negative strap).
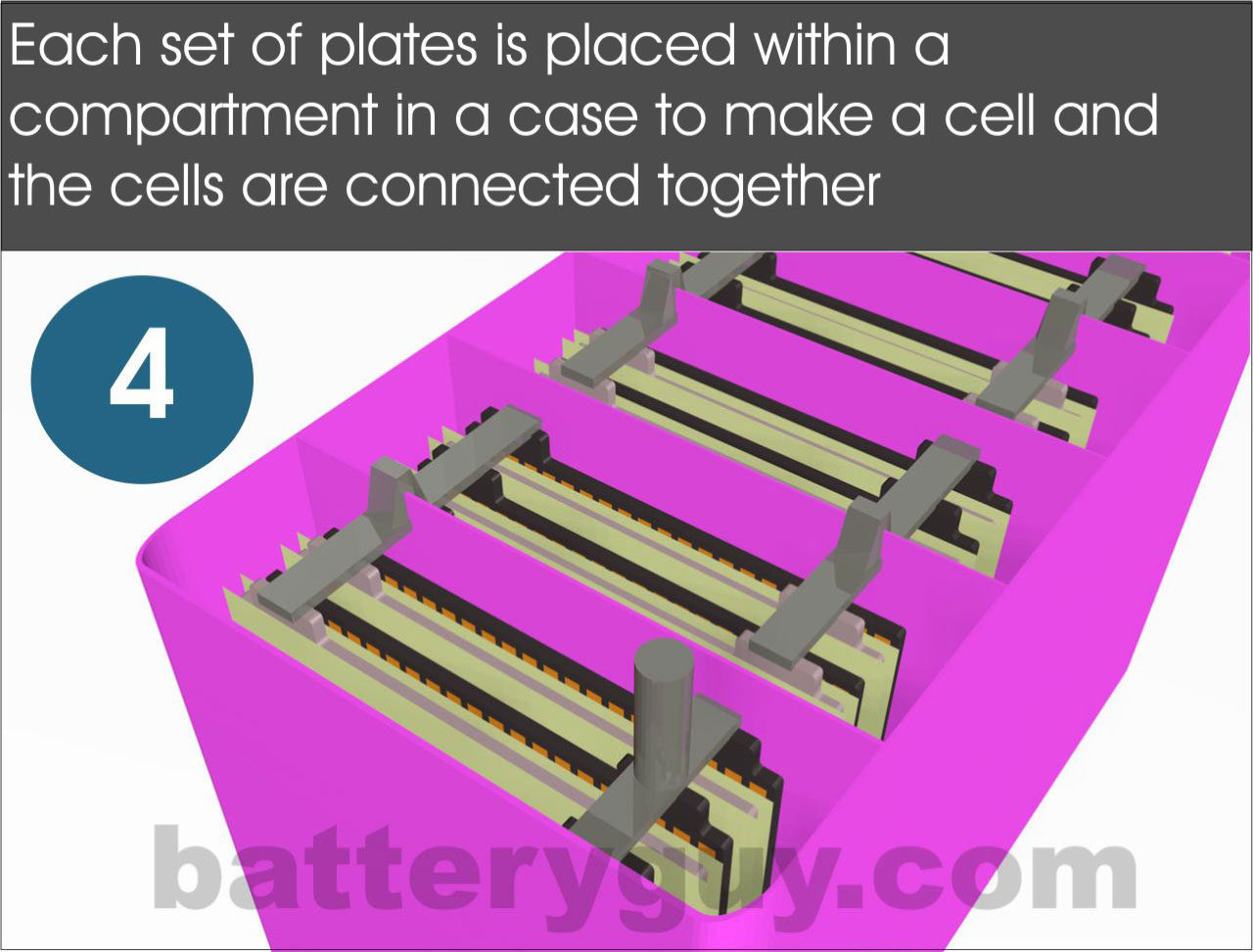
The number of plates may vary but each collection of plates assembled as above is a cell. The battery case itself is divided into compartments to take one cell each.The cells are then connected to each other in series by joining the positive strap of one cell to the negative strap of the next cell with a cell connector.
The battery case is deeper than the lead plates. This is because as the battery is used bits of the active material (paste) fall off the plates and build up at the bottom of the battery. If there was no space for them to do so they would cause a connection between the positive and negative plates, the cell would short out and the battery would die.
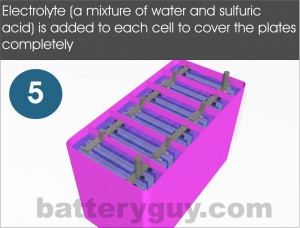
The compartments of the case are then filled with electrolyte – a solution of water and sulfuric acid – until the plates are completely covered. A lid and external terminals are added. Lids vary in design. Some have plugs which can be opened so the electrolyte can be topped up while others will just have a safety valve to release any excess gas that could build up if, for example, the battery is overcharged.
The battery is then left to allow the electrolyte to soak into the paste – a process known as ‘soaking‘ or ‘pickling‘ – before being attached to an electrical supply to charge – known as ‘forming‘.
Lead acid battery types
Wet cell or flooded batteries are the ones described above where the electrolyte is a liquid solution. These are popular as they are cheapest option available due to their low manufacturing costs. Traditionally they came with removable vents or caps in the lid so electrolyte levels could be topped up. Later ‘maintenance free’ batteries were introduced which were designed to prevent liquid loss by containing gases created during normal operation and then converting these back into liquid later. Maintenance free batteries still have vents to release excessive gas build up caused by such events as over charging which may otherwise cause the battery to explode.
However even though some flooded batteries are effectively sealed they should not be confused with the terms Sealed Lead Acid (SLA) or valve-regulated lead-acid (VRLA). These refer to batteries where the electrolyte is not in liquid form – the two most common types are Gel and Absorbent Glass Mat (AGM).
- Gel batteries are a type of sealed lead acid (SLA) where the electrolyte is made up of sulfuric acid and silica to form a jelly like solution that gradually dries out and holds the plates with their paste in place.Gel batteries are more expensive to produce than flooded versions but cheaper than Absorbent Glass Mat.
- Absorbent Glass Mat (AGM) are sealed lead acid (SLA) batteries where a fiberglass mat is impregnated with sulfuric acid and placed between the plates.
Sealed Lead Acid (SLA) / Valve-Regulated Lead-Acid (VRLA) vs Wet Cell/Flooded
The way electrolyte is stored in a sealed lead acid battery means that they have a number of advantages over the older wet cell/flooded design:
- There is no liquid to spill or leak so the batteries are easier to ship and can be mounted at angles.
- They are better at delivering power. Manufacturers of deep cycle flooded batteries often recommend a 4:1 ratio between the amp hour capacity and the largest load it will have to handle while for sealed lead acid this drops to 3:1 which saves space.
- As the plates are held in place:
- they need less of their own strength and so can be made of a purer lead which improves the power to size ratio.
- they are less prone to buckle during deeper discharges so the batteries can be used for deep cycle applications such as powering emergency lights, electric wheelchairs, etc.
- they are less likely to touch and short out in high vibration applications such as sports vehicles or yachts.
- No space is needed below the plates for the collection of active material that gradually flakes off the plates in a wet cell so the power to size ratio improves further.
- They can operate underwater making them popular with marine applications.
- They offer better performance at lower temperatures due to the stabilized electrolyte found in the absorbent glass matt design of the battery, slowing down the chemical reaction within the battery.
- They can be recharged faster and more efficiently. Flooded batteries convert 15-20% of electrical energy from a charger into heat instead of stored power, Gel cells convert 10-16% while the best AGMs lose just 4%. The design of the Absorbent Glass matt (AGM) in the the sealed lead acid battery allows for faster charge times. Because the glass matt absorbs and immobilises the electrolyte available to the plates it allows a faster reaction between the plate material and the electrolyte. The AGM battery has extremely low internal electrical resistance. This, combined with faster acid migration, allows the AGM batteries to deliver and absorb higher rates of amperage than other sealed batteries. True gel batteries have a lower peak charge voltage due to bubbles that can occur in the gel and cause damage, the lower peak charge voltage slowing their overall charge time.
- They suffer less from sulfation because they contain less antimony alloy, lowering the internal discharge of the battery from 8% and 40% with Wet cell/ flooded batteries to 2% and 10% a month with Sealed Lead Acid (SLA). Wet Cell/ flooded batteries with their cavities inside for electrolyte use a lead-antimony alloy to increase mechanical strength. SLA batteries do not require mechanical strength like Wet Cell batteries due to their (AGM) mat material. Usually calcium is alloyed with the lead to reduce gassing and the internal discharge rate
- They discharge at a lower rate when not in use. Flooded batteries discharge at a rate of about 1% per day compared to 1-3% per month from sealed lead acid units.
- They vent little or no gas under normal usage because they operate under pressure which helps recombine the hydrogen and oxygen back into water so they can be placed in enclosed spaces with low ventilation.
Disadvantages including:
- higher costs of manufacture.
- being easier to damage when overcharged.
- causing burn out in alternators designed to charge flooded cell batteries.
- shorter lives – The standard lifespan for SLA batteries is three to five years; for wet-cell batteries it’s up to 20 years.
There is also a small difference between AGM and Gel during their lives. The capacity of AGM batteries tends to decline gradually while Gel batteries maintain their capacity until relatively close to the end.
AGM batteries are preferred when a high burst of amps may be required. Although they are more expensive than flooded versions their flexibility and lower price point compared to Gel’s (see below) makes them popular in standby applications such as emergency lights, electric wheelchairs and smaller UPS systems.
Gel batteries excel in slow discharge rate situations and slightly higher ambient operating temperatures. They are popular as starter batteries for motorcycles, sports vehicles and handle situations where temperature varies widely.
Lead acid battery ratings
Lead acid batteries carry a number of standard ratings which were set up by Battery Council International to explain their capacity:
Cold Cranking Amps (CCA) – how many amps the battery, when new and fully charged, can deliver for 30 seconds at a temperature of 0°F (-18°C) while maintaining at least 1.2 volts per cell (7.2 volts for a 12 volt battery). This is important for starter batteries where the battery must deliver a large amount of power to turn an engine.
Cranking Amps (CA) is the same measurement as Cold Cranking Amps but at 32°F (0°C).
Reserve Capacity (RC) is the number of minutes a new and fully charged battery can discharge 25 amps before the battery drops below 1.75 volts per cell (10.5 volts for a 12 volt battery).
Amp Hour (Ah) ratings are usually found on deep cycle batteries and are an indication of how much power the battery can store. The most common is the ’20 hour rating’ which you will often come across when batteries are being compared. A 100Ah rating means the battery will be able to power a 5 amp appliance for 20 hours ( 5 x 20 = 100) before the cell voltage drops below 1.75 volts per cell ( 10.5 volts for a 12 volt battery).
However it does not mean the battery can power a 50 amp appliance for 2 hours due to Peukert’s Law which states that the faster you discharge a battery, the less capacity it actually has. Peukert’s Law is a mathematical calculation which can be used to work this out. When applied it will show, for example, that a battery with a 100Ah rating will last just over 7 hours when powering a 10 amp appliance.
Peukert’s Law may be useful but it has real world drawbacks. Batteries rarely power one appliance consistently for such a long time period. As such the Amp Hour rating is most useful for general comparisons of one battery over a another – the greater the rating, the more power the battery can store. You can try it out with our calculator in the article Peukert’s Law – how long an amp hour battery will last under a given load.
Watts/Cell is often used for high rate discharge batteries that need to provide substantial power over a short period (usually about 30 minutes). These battery types are often used as backups for computer systems or other critical appliances. Watts/Cell give an indication of how much power the battery can provide in these circumstances rather than how long it is expected to last under a given load.




Leave A Comment?