
Nickel based batteries were first invented over 100 years ago when the only alternative was lead acid and are so called because of their use of nickel metals in the electrodes (see Basic structure of a Nickel battery below). In the 20th century they established a name for themselves as tough, robust and functional – powering everything from small hand held devices to aircraft starter motors.
Nickel batteries stand out for their long operating life and were used at the start of the twentieth century to power cars when many thought electric vehicles would become the norm and gasoline was a passing fad. A few of these cars remain in museums and some still sport their original batteries … which work!
In more recent years this chemistry has lost out to Lithium based batteries that offer more efficiency and greater power at a similar or lower cost. They have, however, not been completely replaced as they are still far more stable (see Safety issues with lithium batteries), perceived by many as tougher, have a longer operating life and can handle higher temperature extremes.
Basic structure of a Nickel battery
The core of a Nickel battery is made up of:
- A negative electrode.
- A positive electrode.
- A separator to ensure the plates do not touch but porous enough to allow chemical reactions between the two via an electrolyte solution which is usually impregnated into the separator material.
These three elements are wound into a cylindrical shape often referred to as the jelly roll or swiss roll structure.
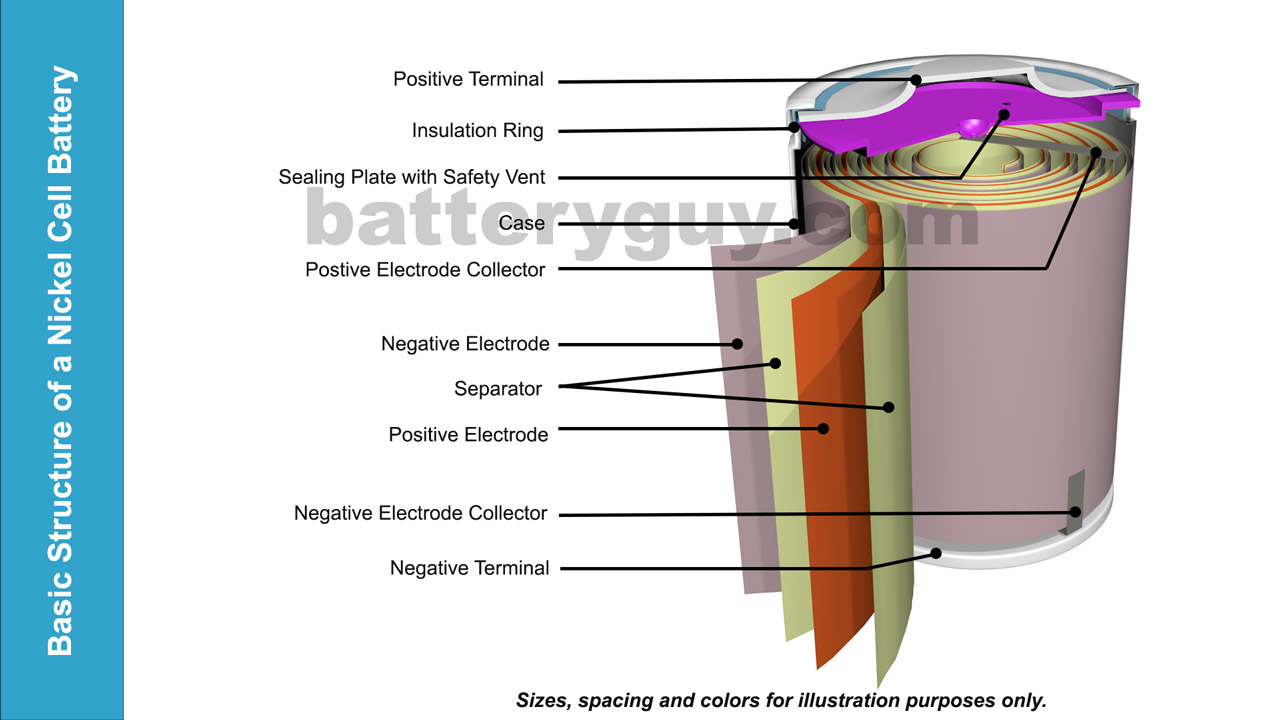
At the bottom of the battery a metal tab connects the negative electrode to the negative terminal, hence the name negative electrode collector. The negative terminal is usually in direct contact with the case of the battery so an insulation ring at the top ensures the positive terminal is isolated from the case.
Also at the top of the battery is another metal tab (known as the positive electrode connector) which connects the positive electrode to a sealing plate. This is in direct contact with the positive terminal and seals in the corrosive electrode but features a self sealing vent which allows gases to escape if the battery malfunctions or is abused through activities such as over or incorrect charging.
(Video of How a Nickel Based Battery is Made with Transcript)
Nickel battery sizes
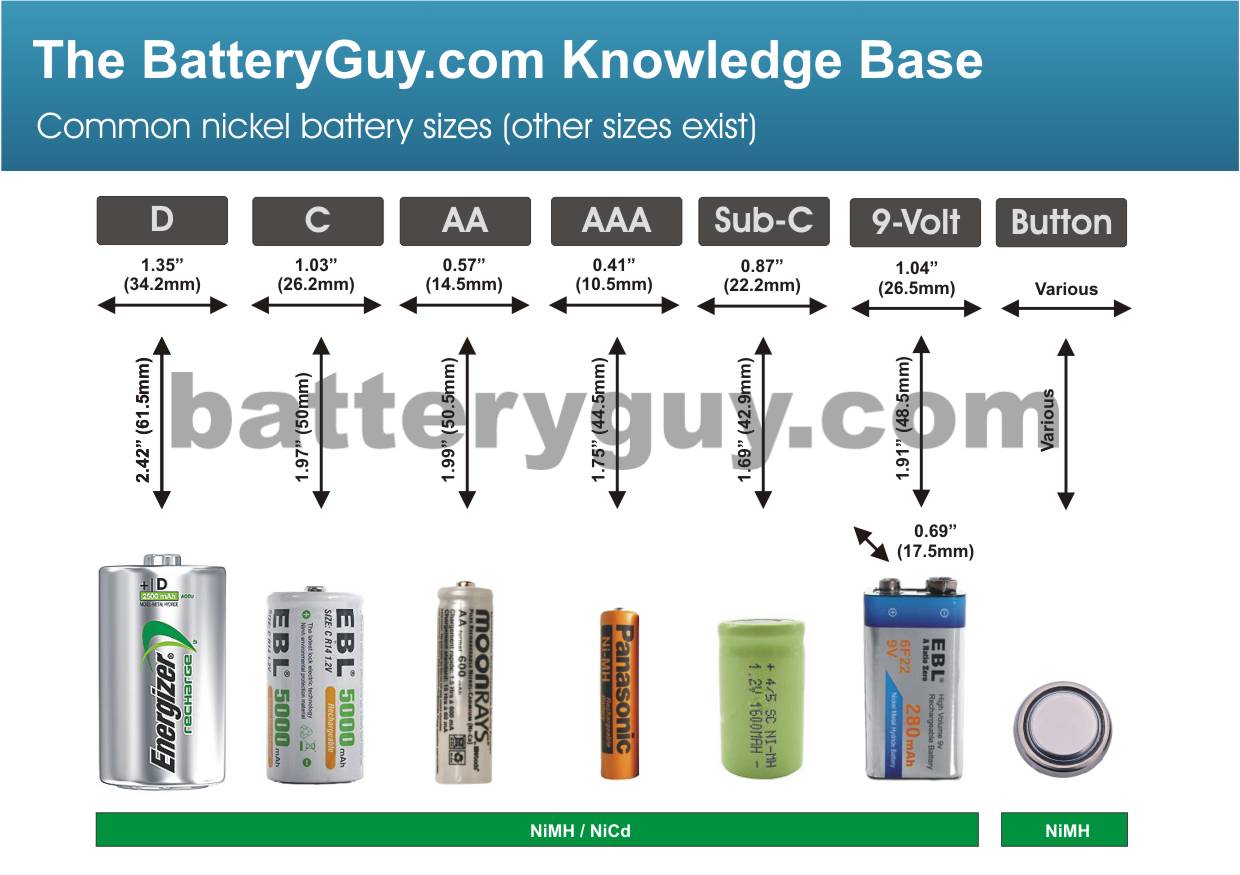
The most common commercially available nickel based battery sizes are:
- D cells
- C cells
- AA cells
- AAA cells
- Sub-C (also known as SC) cells
- 9 Volt
- Button
Nickel battery types
Nickel based batteries come in a variety of chemistries:
- Nickel Iron (NiFe)
- Nickel Zinc (NiZn)
- Nickel Cadmium (NiCd)
- Nickel Metal Hydride
These different chemistries have been developed over the last century but newer technology does not always mean the battery type is ‘better’ in every aspect. All the chemistries developed to date are still in use in various industries or commercially.
Nickel Iron (NiFe)
The first commercially available nickel based battery was Nickel Iron. Patented by Thomas Edison in 1902 it lasts up to four times longer than lead acid and was the battery of choice for electric vehicles at the turn of the century. Although generally seen as having an operating life of 50 years some electric cars produced before the First World War still have their original battery!
Advantages
- Good at handling overcharge and discharge
- Vibration resistant – often used in the mining industry as well as metro systems such as the London Underground and New York Subway
- Long operating life – some units over 100 years old are still operational
Disadvantages
- Low specific energy (50 Wh/kg) – one of the worst ratios in the battery world alongside lead acid
- Poor performance at low temperatures
Nickel Zinc (NiZn)
As gasoline power took over the automotive industry and lead acid was adopted as the battery of choice for engine starting (due to its lower price) nickel based batteries fell into relative obscurity with the exception of some industrial applications such as mining and railroads where they handled vibration better than other alternatives.
However Thomas Edison was not done with Nickel and had patented the Nickel Zinc battery in 1901. With a 1.65 cell voltage and specific energy of 100Wh/kg it offered more power with greater efficiency over a wide temperature range but was let down by a short cycle life and high self-discharge rate.
Advances have been made to address these short comings and some AA consumer units are in production today that use this chemistry but they are rare compared to other nickel based versions.
Nickel Cadmium (NiCd)
The real break through came in the mid 20th century when technological advances lead to the introduction of Nickel Cadmium batteries. It was small enough to power hand held devices such as radios and the first widely available rechargeable cell in a world that had been dominated by disposable zinc cobalt batteries.
Larger versions also became popular in applications such as aircraft starter motors due to their safe stability and tough nature.
However the highly toxic cadmium used by the battery is known to leak from discarded batteries, especially in land fills, and this environmental hazard remained a cloud over an otherwise effective and popular unit.
Advantages
- High cycle life – up to 2,000 discharge/recharge cycles
- Vibration resistant – popular with applications such as power tools
- Long operating life – up to 20 years
- Low temperature friendly – can operate at -40F(-40C)
- Low cost – comparable with Nickel Metal Hydride and slightly more expensive than Lithium
- Good at handling power surges – for such applications as flash photography
- Easy to ship and transport – compared to lithium
Disadvantages
- Low specific energy (up to 80 Wh/kg) – compared to the more recent Nickel Metal Hydride and Lithium based alternatives.
- Contains toxic material – distribution and disposal are heavily regulated in many countries
- High self discharge (10% per month) – compared to Lithium, Alkaline and Lead Acid
- Memory effect myth causes concern – more a perception than a reality (see Nickel Cadmium memory effect – fact or fiction)
Nickel Metal Hydride (NiMH)
In the 1980s nickel technology was improved with the introduction of Nickel metal hydride batteries which offered better specific energy (Wh/kg) and a greater cycle life although it is not as tough and has a higher self-discharge rate.
Its main advantage however was the removal of toxic content and, by offering a new chemistry, it was also able to shake off the memory effect myth (see above).
Advantages
- Recyclable – expensive nickel content makes this viable
- High specific energy – up to 140 Wh/kg as of advances made as recently as 2015, only some Lithium-Ion types perform better.
- Low cost – initially more expensive than Nickel Cadmium it is now broadly similar
- Easy to transport – compared to Lithium
- High cycle life – up to 3,000 cycles
- Operating life – comparable to Lithium but shorter than Alkaline
- No memory effect myth
Disadvantages
- High self discharge – 20% in the first 24 hours and 10% per month thereafter
- Requires a specialist charger
- Voltage – at 1.2 volts per cell it packs less power than Alkaline or Lithium alternatives.
Nickel hydrogen
This chemistry is only used in very specialist applications due to the high cost of production and low specific energy (up to 70 Wh/Kg). Nickel hydrogen is often used in satellites as it can handle temperature extremes and full discharges while offering a long service life.
Nickel Zinc button batteries
Although not hugely popular there are non-rechargeable (primary) nickel based button batteries available and many are manufactured by well known brands such as Varta. Offering a voltage of 1.65 volts they are more power than the 1.55 volts of silver oxide and often found in watches, medical devices, calculators, remote controls, laser pointers, photographic equipment, etc.
Nickel versus Alkaline
Advantages
- Wider operating temperature range
- Much longer cycle life
- Much longer operating life
Disadvantages
- Lower specific energy (Wh/kg)
- Lower cell voltage
- Higher self-discharge
The higher cell voltage of alkaline still makes it more attractive in applications where power is needed fast, such as rapid photography shooting using a flash, but here lithium has proved far superior with most types offering cell voltages of 3.
Thus Alkalines are generally consigned to uses where it is still a leader – low self discharge and a long operating life. This makes it ideal for standby applications such as smoke alarms or in remote controls.
Nickel versus Lithium
Advantages
- Stability
- Wider operating temperature range
Disadvantages
- Higher self-discharge
- Lower specific energy (Wh/kg)
- Lower cell voltage
Nickel’s position as a ‘better’ option to lithium has gradually been eroded over time as lithium’s production costs have fallen and technological advances have improved its cycle life. However nickel based batteries are still seen by many as tougher and safer.


Leave A Comment?